Epigenetic clock tests are probably one of the best methods to assess your overall health, disease risk, mortality risk, and rate of aging.
Epigenetic tests seem to perform far better than genome tests (DNA) tests, which have been somewhat of a disappointment given they are not that insightful to get a better picture of your health, disease and mortality risk (however, I will explain later on why some DNA tests can still be useful).
Biological age versus chronological age
Epigenetic tests or “epigenetic clocks” determine your “biological age”.
Your biological age is your “real age”: it’s how old your body really is. The higher your biological age, the higher your risk of aging-related diseases and dying.
For example, if someone is 50 years old chronologically, but biologically 58 years, it means this person is unhealthier and older for his age, and has a higher risk of aging-related diseases and dying.
Various epigenetic clock tests have been developed (R,R,R,R), and often are further improved upon, while many more new epigenetic clocks are in development.
How do epigenetic clocks work?
Epigenetic clock tests look at the methylation patterns on our DNA.
Parts of our DNA are methylated, while other parts are not methylated.
When DNA is methylated, it means a “methyl group” is put on DNA – a methyl group is a small molecule composed of one carbon atom (C) and 3 hydrogen atoms (Hs).
When a gene is significantly methylated it means it’s covered by a lot of methyl groups, which hinders the copying machinery to reach this gene to translate it into protein. So this gene is “silenced” (in most cases; it’s also possible to activate genes via methylation).
Methylation is an important part of how the epigenome works. It also plays an important role in aging, which also explains why epigenetic clocks are very interesting to measure biological age and rate of aging.
I explain more about the epigenome and its role in aging here.
During aging, our methylation patterns change. We see that large regions of our DNA become less methylated (“hypomethylation”) while specific regions (including regions that encode for gene activity) become overmethylated (“hypermethylation”).
Epigenetic tests look at whether specific regions in the DNA are methylated or not. It can correlate this methylation pattern with your biological age and overall health. People who are epigenetically or biologically older have other methylation patterns than biologically younger or healthier people.
Epigenetic clocks need to be trained on data: methylation data and specific biomarkers of health and disease and mortality to correlate the two.
Researchers need to chart thousands of places in the DNA to see if these places are methylated or not, and then correlate these methylation patterns with health and disease biomarkers, or with chronological age.
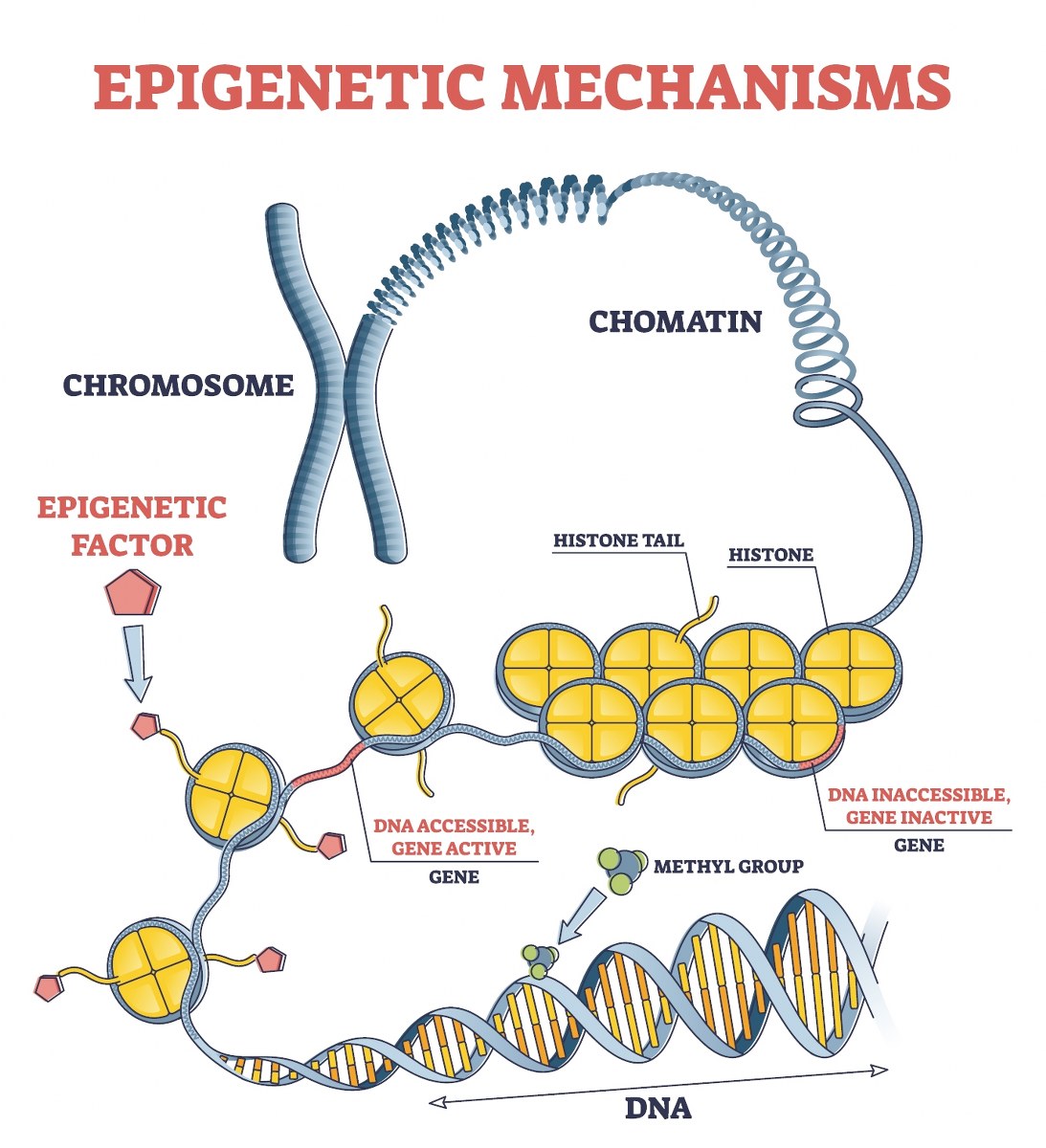
A DNA strand (starting at the bottom) is methylated and then wrapped around histone proteins (yellow structures), which further condense into chromatin, forming chromosomes.
Different types of epigenetic biological clock tests
There exist different kinds of epigenetic clocks. One way to classify them is whether they measure biological age or the rate of aging.
Another way to classify them is whether they are first, second or third generation clocks.
1. Rate of aging clocks
These epigenetic clocks measure how fast your body is currently aging (your rate of aging).
They measure how fast your biological clock is currently ticking.
For example, a result of 0.85 would mean you would only age 0.85 years for each chronological year. That’s good. Unfortunately, many people have a rate of aging test results higher than 1, which means they age too fast.
An example of a rate of aging clock is the DunedinPACE clock (R).
2. Biological age clocks
These clocks measure how old you are biologically. They measure the time on the clock (“how late it is”).
So while “rate of aging clocks” measure how fast the dial is currently ticking, “biological age clocks” measure the current time. The later the time, the older your body is.
Ideally, a good biological age clock measures all the damage your body has accumulated during your lifespan until now.
If you led a generally healthy life, your biological age will be lower than your chronological age.
For example, you will be epigenetically (or biologically) 45 years old despite chronologically being 53 old. This also means that your risk of diseases and dying is lower than what one would expect for your age. That’s good!
Another way to categorize epigenetic tests is by what they actually measure. This leads to the following types of tests:
3. First generation biological age clocks
The first generation of epigenetic clocks correlated methylation patterns with one’s chronological age (R,R).
But why would you want to use the epigenome to find out your chronological age, given you just can find out someone’s chronological age by looking at the birth date on their passport (much cheaper and faster!).
The reason is that chronological age is also a crude biomarker of death and disease. We all know that the older you are chronologically, the higher your risk of getting a heart attack, cancer or dying.
In fact, every 8 years, your risk of dying doubles. So someone who is 38 years old has double the risk of dying than someone who is 30 years old, and by the time you are in your eighties you have a very, very high risk of dying, meaning you can drop dead almost any moment.
So “chronological age” is the easiest biomarker of death and health to come by – everyone knows their chronological age. Therefore, scientists set about correlating epigenetic methylation patterns with one’s biological age.
For this, they correlated epigenetic data (methylation patterns) with the chronological age of thousands of people. This way, they could correlate methylation patterns indirectly with one’s risk of dying (given the higher your chronological age, the higher your risk of dying).
Interestingly, if you then take just one individual, and look at her methylation pattern, one can see how much it deviates from this “average” or “general”’ epigenetic pattern that was found when trained on large groups of people. The more this individual epigenetic pattern differs from the average, the higher or lower the biological age or risk of dying.
For example, if one is 60 years old according to her methylation pattern, while chronologically she is just 53 years old, this means she has double the risk of dying (given every 8 years one risk of dying doubles).
In other words, the “perfect” chronological age clock would always perfectly predict your chronological age. But it doesn’t, given some people will have methylation patterns that make them look younger or older than others (“the average” methylation pattern).
These “chronological age” epigenetic clocks were among the first generation clocks. Examples are the Horvath 2013 clock (R) and Hannum’s clock (R).
So this first generation epigenetic clock tried to predict your risk of dying by predicting your chronological age by looking at methylation patterns. However, better than correlating these clocks with chronological age would be to correlate them to actual mortality or disease risk.
However, mortality data is difficult to come by. One would need to analyze the epigenome from people and wait until they die, which is very costly and time consuming. Or one could look into databases of stored blood (of people of which their birthdate and date of death are known) and epigenetically analyze their blood. But this would also be time consuming and costly.
So scientists set about trying to indirectly measure mortality (and disease) risk. And this brings us to the second type of epigenetic clocks.
4. Second generation biological age clocks
These clocks measure disease or mortality indirectly, for example by looking at specific substances in the blood which are associated with disease risk or mortality risk.
Examples of such substances are glucose levels, CRP levels (a protein associated with inflammation), GDF15 protein levels, TIMP2 protein levels, etc.
Scientists have access to blood samples or data stored in data banks which measured the levels of these substances in the blood of thousands of people.
They then look at which epigenetic patterns are correlated with the blood levels of these substances, which in turn correlate with disease or mortality risk.
For example, people with high levels of glucose have a higher risk of dying. Having higher levels of glucose is also correlated with specific epigenetic patterns. So an epigenetic clock can be trained to figure out which epigenetic patterns are correlated with higher levels of glucose, and thus also with a higher risk of dying.
Examples of these second generation clocks are Horvath’s DNAmGrimAge clock (R,R), which has been trained to look at methylation patterns correlating with seven blood biomarkers, namely levels of adrenomedullin, beta‐2 microglobulin, cystatin C, growth differentiation factor 15, leptin, plasminogen activation inhibitor 1 (PAI-1), and tissue inhibitor metalloproteinase 1 (TIMP1). This clock also takes into account some other markers of health and mortality, like smoking and sex.
Another clock is the Horvath-Levine’s DNAm PhenoAge clock (R), which looks at 9 blood biomarkers (creatine, CRP, red blood cell distribution width (RBCDW), total leukocytes, lymphocytes, albumin, glucose, mean cell volume, alkaline phosphatase) and some other biomarkers of health, disease and mortality.
These second generation clocks have been shown to more accurately predict mortality risk and disease risk than the first generation chronological age clocks.
Of course, this is a bit of a circuitous and indirect way of measuring health and disease and mortality risk. A more direct way would be to actually measure how healthy you are.
This brings us to the third generation of epigenetic clocks.
5. Third generation biological age clocks
These clocks take into account various biomarkers of disease and health, such as grip strength, brain size, walking speed, dental health, cognitive functioning, blood tests (e.g. measuring Hb1ac, LDL and HDL cholesterol), and so on.
These biomarkers, especially when taken together, paint a much better picture of one’s overall health and mortality risk than just looking at a few blood proteins or your chronological age, like the earlier generation epigenetic tests.
Scientists then try to figure out which epigenetic patterns correlate with these biomarkers. This way, they then can just look at the epigenetic pattern of an individual to predict their health or mortality risk without having to actually measure all these biomarkers.
Examples of such clocks is the DunedinPACE clock (R).

What is the best epigenetic biological age test?
The best epigenetic clock would be one that is trained on many powerful biomarkers of health, disease and mortality risk.
These can be real, physiological biomarkers, like the size of your brain (during aging, the brain shrinks), blood pressure, weight, dental health, facial health (if you look older, changes are your body is also older), balance, grip strength, gait speed, working memory, reasoning, and so on. It should also incorporate other biomarkers, like blood biomarkers (HDL cholesterol, inflammatory proteins, glucose levels, and so on).
One of the few clocks that actually measures various of such biomarkers, is the DunedinPACE clock. This clock has been the result of scientists tracking the health of around a thousand people for decades, regularly measuring their grip strength, dental health, brain size, memory, facial aging, and so on. All these measurements paint a more accurate picture of one’s health and biological age.
The DunedinPACE is a rate of aging clock: it tells how fast your biological clock is currently ticking. That’s good.
However, you would also want to know the time on the clock (how much time has already passed). For this, you need a biological age clock. A decent biological age clock is the GrimAge clock (a second generation epigenetic clock).
So ideally, one does two epigenetic tests: one test to measure your rate of aging (how fast the clock is ticking now), and another to measure your biological age (the time on the clock).
Other things you would preferably like to see in a good epigenetic clock:
- Ideally, the clock is trained on data of large groups of people, like thousands of people or more. The larger the testing group, the more accurate the clock can be.
- Ideally, the epigenetic clock is published in peer-reviewed journals, and its data and algorithms are open source so the clock can be checked by other, independent scientists. Various epigenetic clock companies do the opposite: they developed in-house epigenetic clocks and do not share their data and algorithms with other scientists, so it’s more difficult to see how these clocks work, and if they are actually accurate and predictive.
- They have high test-retest results. This means if you use these clocks to measure one’s biological age, and then quickly do this test again, the results should be very similar. Currently, some epigenetic clocks have low test-retest results: this means when you take this test and then retest an hour or day later, the results can differ wildly, which is not good of course.
- Proper epigenetic clocks are correlated with both disease and mortality risk. So they tell you your risk of diseases, and your risk of dying.
- They use state of the art machine learning and/or mathematical and statistical methods to properly correlate epigenetic patterns with health, aging and mortality. Lots of earlier clocks use more simple methods, like linear regression, while newer clocks use machine learning and improved statistical methods.
The future of biological age clocks
Currently, many new aging clocks are being developed. Instead of only looking at methylation patterns, some novel clocks will also focus on other forms of epigenomic organization, like histonylation (histones are proteins around which the DNA is wrapped).
Other clocks will try to chart aging in different tissues. After all, not all our tissues and organs age in the same way. For example, breast tissue and ovaries seem to age faster than other tissues.
But likely, the best aging clock will be an incorporate different kinds of clocks, not just epigenetic clocks, but also clocks that look at proteins (proteomic clocks), metabolites (metabolic clocks), RNA (transcriptomic clocks), and the microbiome of the mouth and gut (microbiomic clocks) for example.
Too much information? Or want to learn more?
Dr. Verburgh’s new upcoming book on longevity will likely best book on longevity for the general public.
To be published in a few months.
Subscribe to our newsletter to stay updated
(sent out every month or two months)